In addition to technology, the synthesis of glycosides has always been of interest to science, as it is a very common reaction in nature. Recent papers by Schmidt and Toshima and Tatsuta, as well as many references cited therein, have commented on a wide range of synthetic potentials.
In the synthesis of glycosides, a multi-sugar components is combined with a nucleophiles,such as alcohols,carbohydrates, or proteins, if a selective reaction with one of the hydroxyl groups of a carbohydrate in required, all other functions must be protected in the first step. In principle, enzymatic or microbial processes, due to their selectivity, can replace complex chemical protection and deprotection steps to selectively from glycosides in regions. However, due to the long history of alkyl glycosides, the application of enzymes in the synthesis of glycosides has not been widely studied and applied.
Due to the capacity of suitable enzyme systems and high production costs, enzymatic synthesis of alkyl polyglycosides is not ready to be upgraded to the industrial level, and chemical methods are preferred.
In 1870,M.A.colley reported the synthesis of “acetochlorhydrose”(1,figure2) by reaction of dextrose(glucose)with acetyl chloride, which eventually led to the history of glycoside synthesis routes.
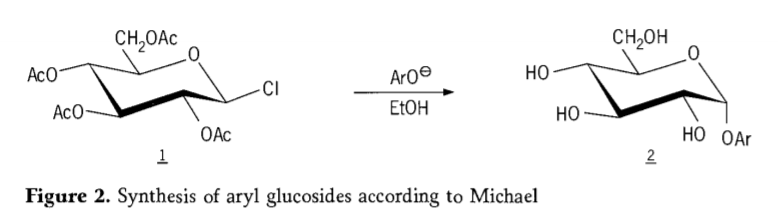
Tetra-0-acetyl-glucopyranosyl halides(acetohaloglucoses) were later found to be useful intermediates for the stereoselective synthesis of pure alkyl glucosides. In 1879, Arthur Michael succeeded in preparing definite,crystallizable aryl glycosides from Colley’s intermediates and phenolates. (Aro-,Figure 2).
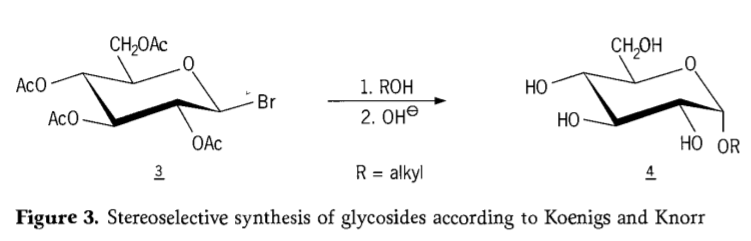
In 1901,Michael’s synthesis to a broad range of carbohydrates and hydroxylic aglycons, when W.Koenigs and E.Knorr introduced their improved stereoselective glycosidation process(Figure 3). The reaction involves an SN2 substitution at the anomeric carbon and proceeds stereoselectively with inversion of configuration, producing for example the α-glucoside 4 from the β-anomer of the aceobromoglucose intermediate 3. The Koenigs-Knorr synthesis takes place in the presence of silver or mercury promotors.
In 1893, Emil Fischer proposed a fundamentally different approach to the synthesis of alkyl glucosides. This process is now well known as the “Fischer glycosidation” and comprises an acid-catalyzed reaction of glycoses with alcohols. Any historical account should nevertheless also include A.Gautier’s first reported attempt in 1874,to convert dextrose with anhydrous ethanol in the presence of hydrochloric acid. Due to a misleading elemental analysis, Gautier believed he had obtained a “diglucose”. Fischer later demonstrated that Gautier’s “diglucose” was in fact mainly ethyl glucoside (Figure 4).
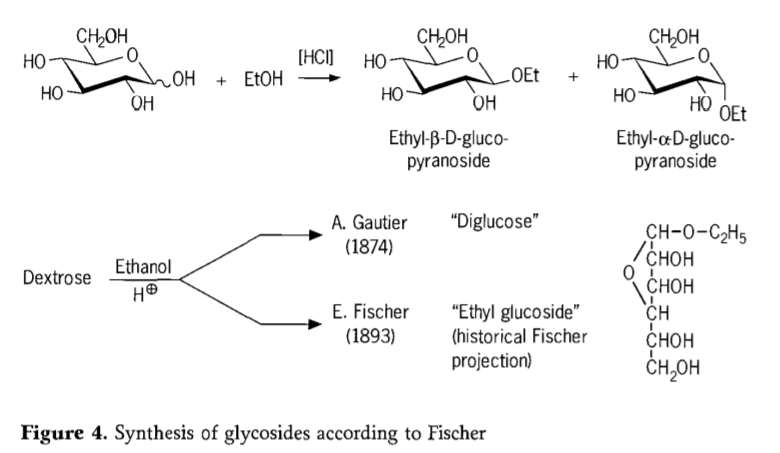
Fischer defined the structure of ethyl glucoside correctly, as may be seen from the historical furanosidic formula proposed. In fact, Fischer glycosidation products are complex, mostly equilibrium mixtures of α/β-anomers and pyranoside/furanoside isomers which also comprise randomly linked glycoside oligomers.
Accordingly, individual molecular species are not easy to isolate from Fischer reaction mixtures, which has been a serious problem in the past. After some improvement of this synthesis method, Fischer subsequently adopted the Koenigs-Knorr synthesis for his investigations. Using this process, E.Fischer and B.Helferich were the first t report the synthesis of a long-chain alkyl glucoside exhibiting surfactant properties in 1911.
As early as 1893,Fischer had correctly noticed essential properties of alkyl glycosides, such as their high stability towards oxidation and hydrolysis, especially in strongly alkaline media. Both characteristics are valuable for alkyl polyglycosides in surfactant applications.
Research related to the glycosidation reaction is still ongoing and several interesting routes to glycosides have been developed in the recent past. Some of the procedures for the synthesis of glycosides are summarized in Figure 5.
In General, chemical glycosidation processes may be divided into processes leading to complex oligomer equilibria in acid-catalysed glycosyl exchange.
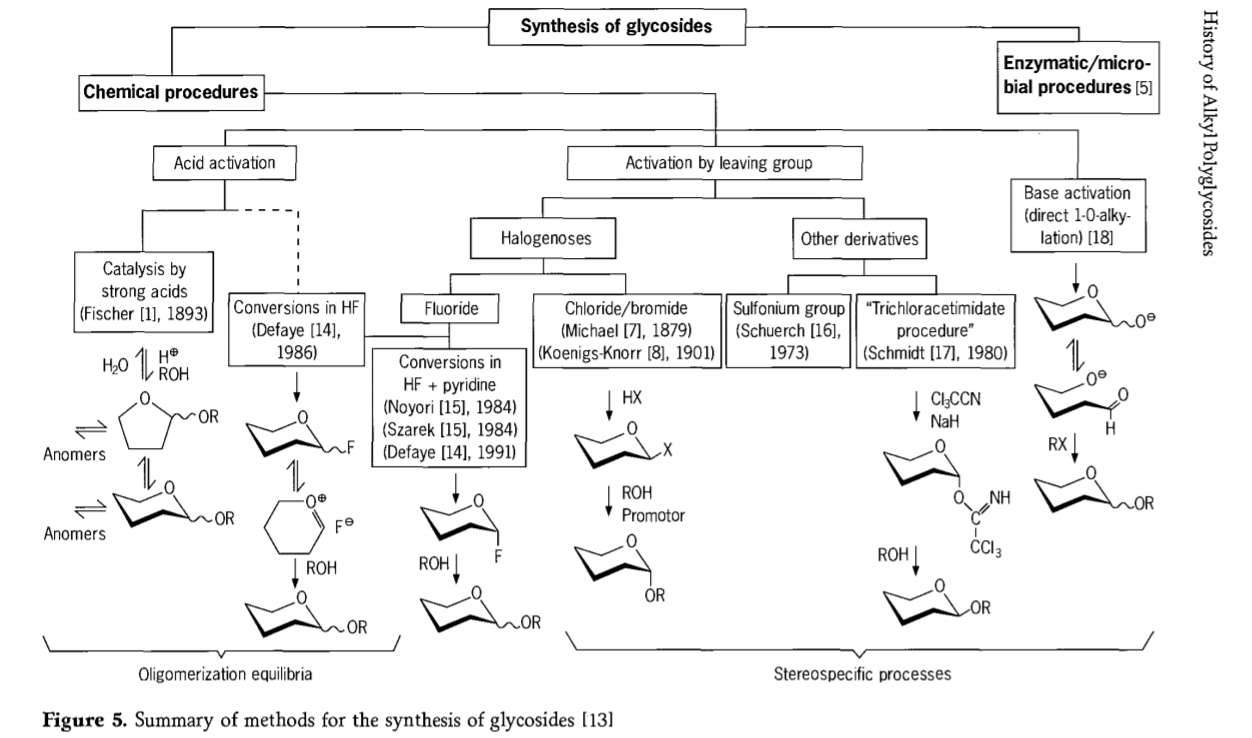
Reactions on appropriately activated carbohydrate substrates(Fischer glycosidic reactions and hydrogen fluoride(HF) reactions with unprotected carbohydrate molecules) and kinetics controlled, irreversible, and mainly stereotaxic substitution reactions. A second type of procedure may lead to the formation of individual species rather than in complex mixtures of reactions, especially when combined with conservation group techniques. Carbohydrates may leave groups on the ectopic carbon, such as halogen atoms, sulfonyls, or trichloroacetimidate groups, or be activated by bases prior to conversion to triflate esters.
In the particular case of glycosidations in hydrogen fluoride or in mixtures of hydrogen fluoride and pyridine (pyridinium poly [hydrogen fluoride]),glycosyl fluorides are formed in situ and are smoothly converted into glycosides, for example with alcohols. Hydrogen fluoride was shown to be a strongly activating, nondegrading reaction medium; equilibrium auto condensation(oligomerization) is observed similar to the Fischer process, although the reaction mechanism is probably different.
Chemically pure alkyl glycosides are only suitable for very special applications. For example, alkyl glycosides have been used successfully in biochemical research for the crystallization of membrane proteins, such as the three dimensional crystallization of porin and bacteriorhodopsin in the presence of octyl β-D-glucopyranoside(further experiments based on this work lead to the Nobel prize in chemistry for Deisenhofer, Huber and Michel in 1988).
During the course of the development of alkyl polyglycosides, stereoselective methods have been used on a laboratory scale to synthesize a variety of model substances and to study their physicochemical properties, due to their complexity, the instability of intermediates and the amount and critical nature of process wasters, syntheses of the Koenigs-Knorr type and other protective group techniques would create significant technical and economic problems. Fischer-type processes are comparatively less complicated and easier to carry out on a commercial scale and accordingly, are the preferred method for the production of alkyl polyglycosides on a large scale.
Post time: Sep-12-2020





